Trajenta 5 mg, 30 tablets
501£
View analogsТражента показана взрослым с сахарным диабетом 2 типа в качестве дополнения к диете и физическим упражнениям для улучшения гликемического контроля.
Buy
Product quantities
Form of Release: Tablets
Product Brand: Egyptian pharmaceutical trading company
Product Categories: Diabetes
Trade Name:
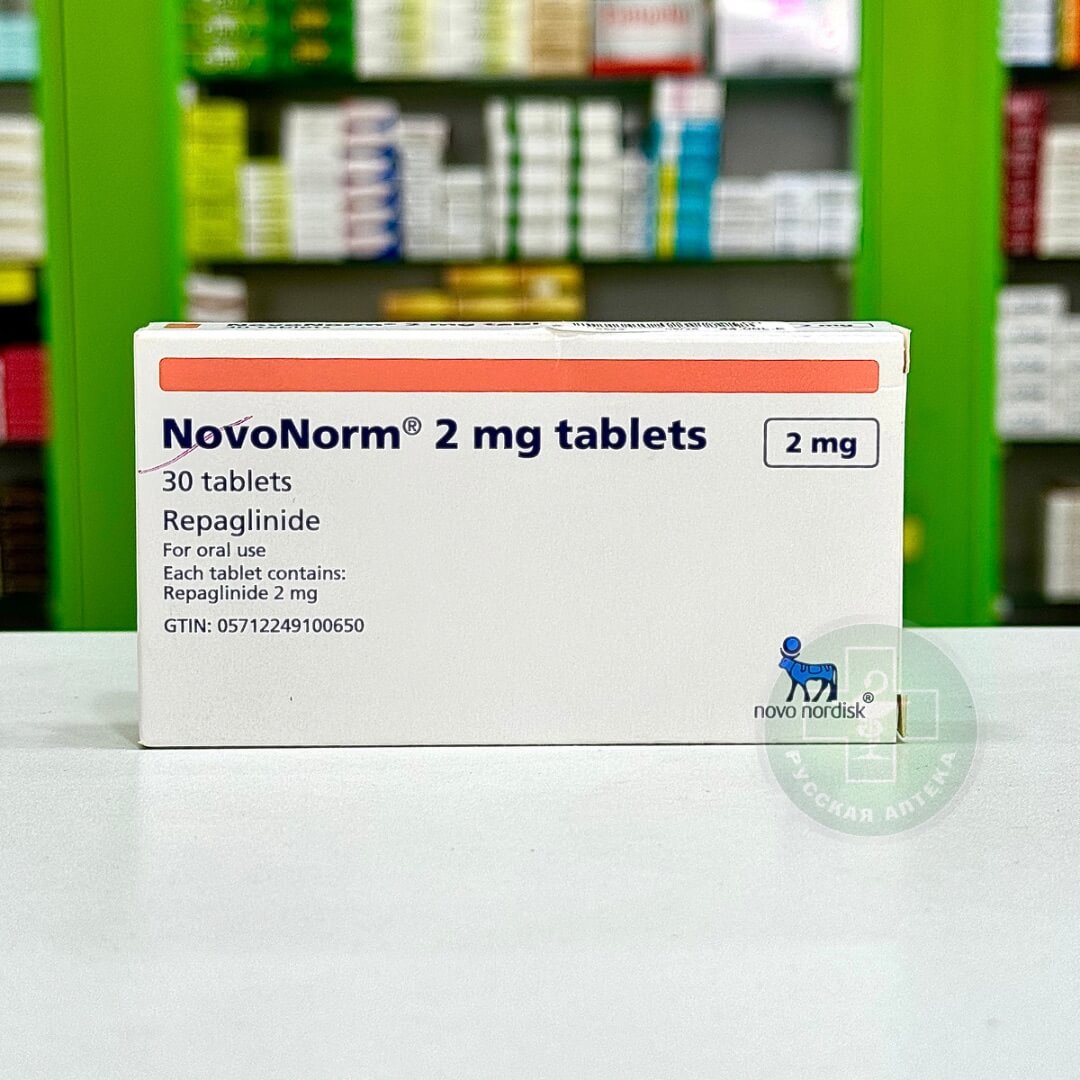
Trajenta
Linagliptin 5 mg
30 Film-coated tablets
Composition:
Linagliptin 5 mg
Inactive ingredients:
Mannitol, Pregelatinised starch (maize), Maize starch, Copovidone, Magnesium stearate, Hypromellose, Titanium dioxide (E171), Talc, Macrogol (6000), Iron oxide red (E172)
Properties:
Pharmacotherapeutic group: Drugs used in diabetes, dipeptidyl peptidase 4 (DPP-4) inhibitors.
Linagliptin is an inhibitor of the enzyme DPP-4 (dipeptidyl peptidase 4, EC 3.4.14.5) an enzyme which is involved in the inactivation of the incretin hormones GLP-1 and GIP (glucagon-like peptide1, glucose-dependent insulinotropic polypeptide). These hormones are rapidly degraded by the enzyme DPP-4. Both incretin hormones are involved in the physiological regulation of glucose homeostasis.
Incretins are secreted at a low basal level throughout the day and levels rise immediately after meal intake. GLP-1 and GIP increase insulin biosynthesis and secretion from pancreatic beta cells in the presence of normal and elevated blood glucose levels. Furthermore GLP-1 also reduces glucagon secretion from pancreatic alpha cells, resulting in a reduction in hepatic glucose output. Linagliptin binds very effectively to DPP-4 in a reversible manner and thus leads to a sustained increase and a prolongation of active incretin levels. Linagliptin glucose-dependently increases insulin secretion and lowers glucagon secretion thus resulting in an overall improvement in the glucose homeostasis.
Indications:
Trajenta is indicated in adults with type 2 diabetes mellitus as an adjunct to diet and exercise to improve glycaemic control asmonotherapy:
- when metformin is inappropriate due to intolerance, or contraindicated due to renal impairment combination therapy;
- in combination with other medicinal products for the treatment of diabetes, including insulin, when these do not provide adequate glycaemic control;
Dosage and administration:
-The dose of linagliptin is 5 mg once daily. -When linagliptin is added to metformin, the dose of metformin should be maintained, and linagliptin administered concomitantly.
-When linagliptin is used in combination with a sulphonylurea or with insulin, a lower dose of the sulfonylurea or insulin, may be considered to reduce the risk of hypoglycaemia;
-Renal impairment:
For patients with renal impairment, no dose adjustment for linagliptin is required.
-Hepatic impairment:
Pharmacokinetic studies suggest that no dose adjustment is required for patients with hepatic impairment but clinical experience in such patients is lacking.
-Elderly:
No dose adjustment is necessary based on age.
-Pediatric population:
A clinical trial did not establish efficacy in pediatric patients 10 to 17 years of age.Therefore, treatment of children and adolescents with linagliptin is not recommended. Linagliptin has not been studied in pediatric patients under 10 years of age.
-The tablets can be taken with or without a meal at any time of the day. If a dose is missed, it should be taken as soon as the patient remembers. A double dose should not be taken on the same day.
Side effects:
-Infections and infestations: Nasopharyngitis uncommon
-Immune system disorders:
Hypersensitivity(bronchial hyperreactivity)
-Metabolism and nutrition disorders:
Hypoglycemia
-Respiratory, thoracic and mediastinal disorders: cough
-Gastrointestinal disorders:
Pancreatitis
Constipation
-Skin and subcutaneous tissue disorders:
Angioedema
Urticaria
Rash
Bullous pemphigoid
-Analysis:
Amylase increased
Lipase increased
Contraindications:
-Hypersensitivity to the active substance or to any of the excipients
Warnings and precautions:
- Linagliptin should not be used in patients with type 1 diabetes or for the treatment of diabetic ketoacidosis.
- Patients should be informed of the characteristic symptoms of acute pancreatitis. If pancreatitis is suspected, Trajenta should be discontinued; if acute pancreatitis is confirmed, Trajenta should not be restarted. Caution should be exercised in patients with a history of pancreatitis.
- Pregnancy:
The use of linagliptin has not been studied in pregnant women. Animal studies do not indicate direct or indirect harmful effects with respect to reproductive toxicity. As a precautionary measure, it is preferable to avoid the use of linagliptin during pregnancy.
- Breast-feeding:
A risk to the breast-fed child cannot be excluded. A decision must be made whether to discontinue breast-feeding or to discontinue/abstain from linagliptin therapy taking into account the benefit of breast-feeding for the child and the benefit of therapy for the woman.
- Fertility:
No studies on the effect on human fertility have been conducted for linagliptin.
- Effects on ability to drive and use machines:
Linagliptin has no or negligible influence on the ability to drive and use machines. However patients should be alerted to the risk of hypoglycaemia especially when combined with sulphonylurea and/or insulin.
- Keep out of reach of children.
Storage:
Store in a cool dry place.
Package:
Carton box holds 3 strips each of 10 film-coated tablets and paper instructions
Tags, Keywords:
Active Ingredients:
No comments yet. Be the first to write one.
Your comment will be published after moderation.
You’ll receive a notification to your email when someone replies.
 Русский
Русский English
English عربي
عربي