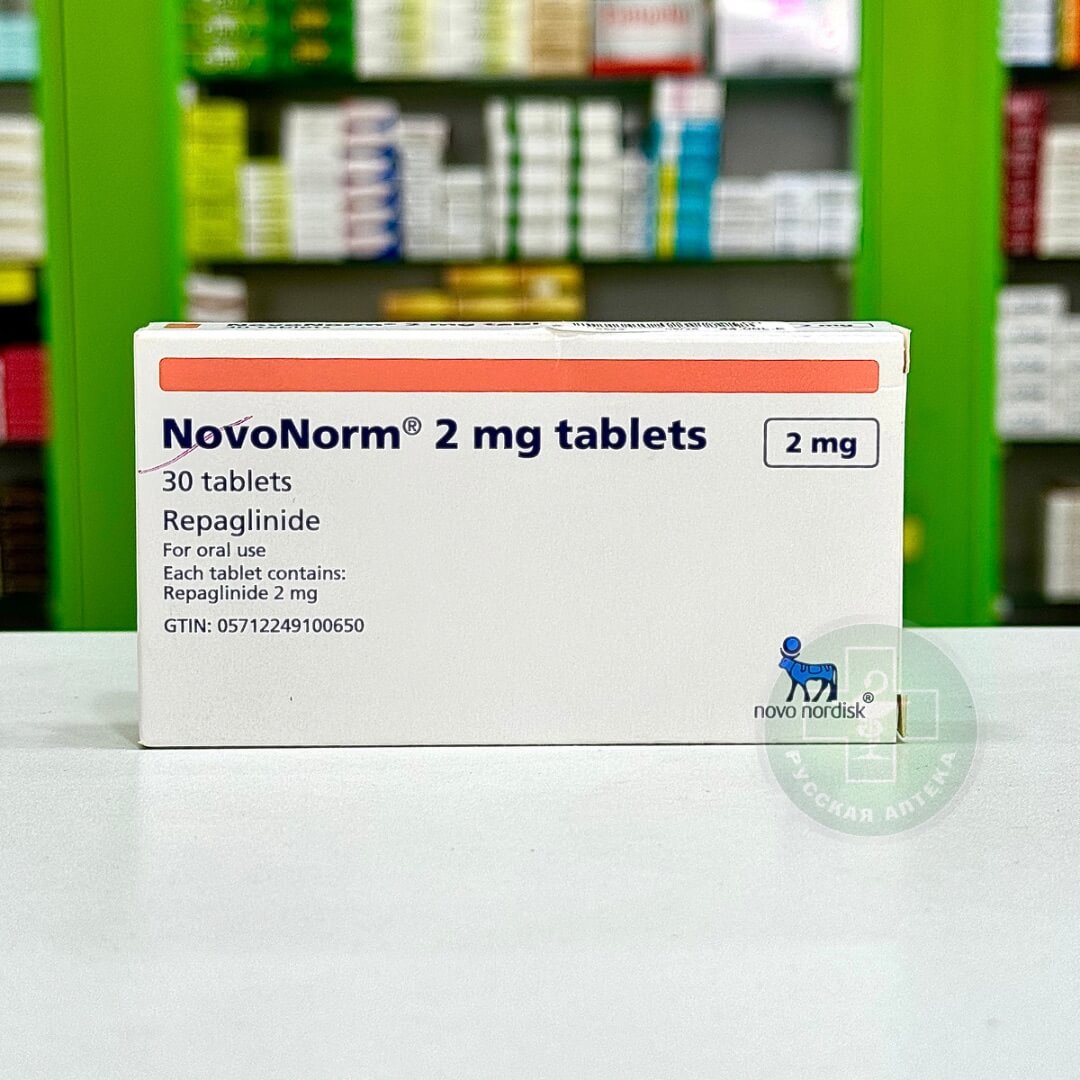
NovoNorm 2 mg 30 tab
44£
View analogs- Type 2 diabetes mellitus (if diet therapy, weight loss and exercise are ineffective) in monotherapy or in combination with metformin or thiazolidinediones in cases where it is not possible to achieve satisfactory glycemic control with monotherapy with repaglinide, metformin or thiazolidinediones.
Buy
Product quantities
Form of Release: Tablets
Product Brand: Novo Nordisk
Product Categories: Diabetes
Trade name:
NovoNorm 30 tabl
Ingredients:
Each tablet contains:
Repaglinide 2 mg
Other components:
poloxamer 188, povidone, meglumine, corn starch, anhydrous calcium hydrogen phosphate, microcrystalline cellulose (E460), glycerol 85%, potassium polacriline (potassium polyacrylate), magnesium stearate, red iron oxide.
Description:
Treatment of type 2 diabetes mellitus (if diet therapy, weight loss and exercise are ineffective) in monotherapy or in combination with metformin or thiazolidinediones in cases where it is not possible to achieve satisfactory glycemic control with monotherapy with repaglinide, metformin or thiazolidinediones.
Properties:
Oral hypoglycemic agent. Rapidly reduces blood glucose levels by stimulating the release of insulin from functioning pancreatic B-cells. The mechanism of action is associated with the ability to block ATP-dependent channels in B-cell membranes by acting on specific receptors, which leads to cell depolarization and the opening of calcium channels. After taking repaglinide, an insulinotropic response to food intake is observed within 30 minutes, which leads to a decrease in blood glucose levels. In patients with type 2 diabetes mellitus (non-insulin-dependent), when taking repaglinide in doses from 500 mcg to 4 mg, a dose-dependent decrease in blood glucose levels is observed.
Indications:
- Type 2 diabetes mellitus (if diet therapy, weight loss and exercise are ineffective) in monotherapy or in combination with metformin or thiazolidinediones in cases where it is not possible to achieve satisfactory glycemic control with monotherapy with repaglinide, metformin or thiazolidinediones.
Directions for use and dosage:
Inside. The dosage regimen is set individually, selecting the dose in order to optimize glucose levels. There were no clinically significant differences in the pharmacokinetic parameters of repaglinide when taken immediately before meals, 15 and 30 minutes before meals, or on an empty stomach.
The recommended initial daily dose is 0.5 mg. The average daily dose is 12 mg. The maximum daily dose is 16 mg.
Contraindications:
- Hypersensitivity to repaglinide; type 1 diabetes mellitus, diabetic ketoacidosis, diabetic precoma and coma;
- infectious diseases, major surgical interventions and other conditions requiring insulin therapy;
- severe liver dysfunction; simultaneous use of gemfibrozil;
- pregnancy, breastfeeding period;
- age under 18 and over 75 years.
Precautionary measures:
With caution:
- in case of mild to moderate liver dysfunction, febrile syndrome, chronic renal failure, alcoholism, general serious condition, malnutrition.
- If you have liver or kidney disease, extensive surgery, or a recent illness or infection, the effectiveness of repaglinide may be reduced.
- In debilitated or malnourished patients, repaglinide should be taken in minimal initial and maintenance doses. To prevent hypoglycemic reactions in this category of patients, the dose should be selected with caution.
- The resulting hypoglycemic conditions are usually reactions of moderate severity and are easily relieved by the intake of carbohydrates. In severe conditions, it may be necessary to administer intravenous glucose. The likelihood of developing such reactions depends on the dose, dietary habits, intensity of physical activity, and stress.
Impact on the ability to drive vehicles and machinery:
During the period of use of repaglinide, patients should be careful when driving vehicles and machinery, as well as when engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Side effects:
- From the immune system: very rarely – generalized hypersensitivity reactions; frequency unknown – itching, skin rash, urticaria.
- Metabolism: often – hypoglycemia; frequency unknown – hypoglycemic coma, hypoglycemia with loss of consciousness.
- On the part of the organ of vision: very rarely – visual impairment, especially at the initial stage of therapy (usually transient).
- From the digestive system: often – abdominal pain, diarrhea; very rarely – vomiting, constipation; frequency unknown – nausea, pancreatitis.
- From the liver and biliary tract: increased activity of liver enzymes, severe liver dysfunction, including hepatitis and jaundice.
Other: frequency unknown – alopecia, hemolytic anemia, Stevens-Johnson syndrome.
Storage:
Store at a temperature of 15 to 25 degrees in a dry place.
Package:
The cardboard pack contains 2 blisters of 15 tablets, paper instructions.
Tags, Keywords:
Active Ingredients:
No comments yet. Be the first to write one.
Your comment will be published after moderation.
You’ll receive a notification to your email when someone replies.
 Русский
Русский English
English عربي
عربي