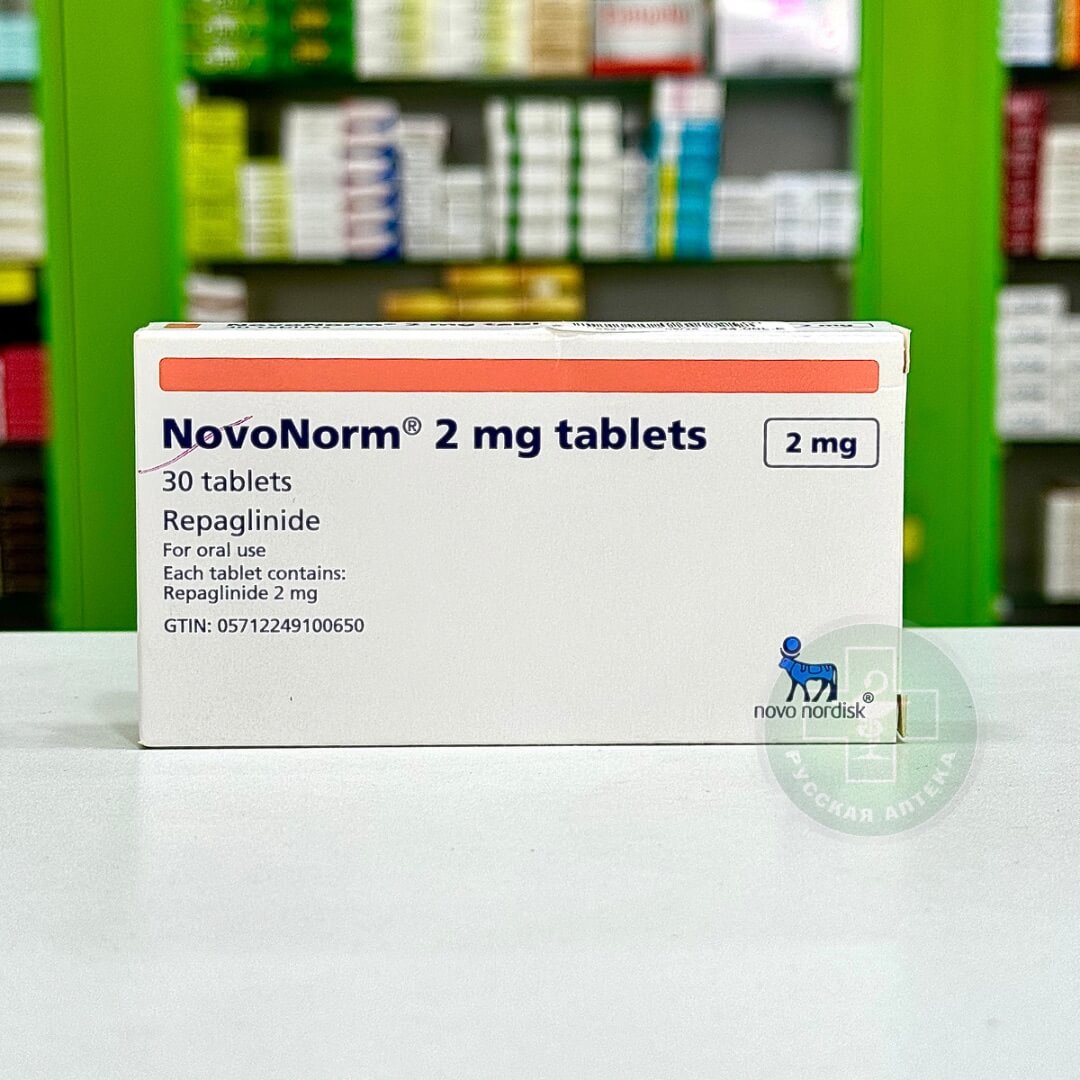
Omegapress 0,1 mg 30 tablets
288£
View analogsOmegapress tablets are indicated for the treatment of:
• cranial diabetes insipidus
• primary nocturnal enuresis in patients from 6 years of age with normal ability to concentrate urine, who are refractory to an enuresis alarm or in whom an enuresis alarm is contraindicated or inappropriate.
Buy
Product quantities
Form of Release: Tablets
Product Brand: Atco pharma
Product Categories: Diabetes
Omegapress 0.1 mg 30 tablets
Composition:
Active Ingredients: Each tablet contains 0.1mg Desmopressin acetate
Excipients: Lactose monohydrate, starch 1500, colloidal silicon dioxide, Magnesium stearate.
Indications:
Omegapress tablets are indicated for the treatment of:
• cranial diabetes insipidus
• primary nocturnal enuresis in patients from 6 years of age with normal ability to concentrate urine, who are refractory to an enuresis alarm or in whom an enuresis alarm is contraindicated or inappropriate.
Dosage and method of administration
There is no predictable dose equivalence between intranasal and oral dosing, so individual dose titration is needed.
For ADH-sensitive Cranial Diabetes Insipidus:
Dosage is individualized in diabetes insipidus but clinical experience has shown that the total daily dose normally lies in the range of 0.2mg to 1.2mg. A suitable starting dose in adults and children is 0.1mg three times daily. The dosage regimen should then be adjusted in accordance with the patient's response.
For the majority of patients, the maintenance dose is 0.1mg to 0.2mg three times daily.
In the event of signs of water retention/hyponatraemia, treatment should be interrupted and the dose should be adjusted
Primary Nocturnal Enuresis:
The recommended initial dose is 0.2mg at bedtime. If this dose is not sufficiently effective, the dose may be increased up to 0.4mg. Fluid intake must be limited to a minimum from 1 hour before until 8 hours after administration. In the event of signs or symptoms of water retention and/or hyponatraemia (headache, nausea/vomiting, weight gain, and, in severe cases, convulsions) treatment should be interrupted until the patient has fully recovered. When restarting treatment, strict fluid restriction should be enforced.
Omegapress Tablets are intended for treatment periods of up to 3 months. The need for continued treatment should be reassessed by means of a period of at least one week without omegapress Tablets.
Contraindications
• Habitual or psychogenic polydipsia (resulting in a urine production exceeding 40
mL/kg/24 hours)
• A history of known or suspected cardiac insufficiency and other conditions requiring treatment with diuretics
• Moderate and severe renal insufficiency (creatinine clearance below 50 mL/min)
• Known hyponatraemia
• Syndrome of inappropriate anti-diuretic hormone secretion (SIADH)
• Hypersensitivity to desmopressin acetate or any of the excipients of omegapress
Precautions:
-When used for primary nocturnal enuresis, the fluid intake must be limited to a minimum from 1 hour before administration, until the next morning (at least 8 hours) after administration.
-Treatment without concomitant reduction of fluid intake may lead to water retention and/or hyponatraemia with or without accompanying warning signs and symptoms (headache, nausea/vomiting, weight gain and, in severe cases, convulsions).
-In the event of signs of water retention/hyponatraemia in cranial diabetes insipidus patients, treatment should be interrupted and the dose should be adjusted.
-This product contains lactose. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicine.
-Severe bladder dysfunction and outlet obstruction should be considered before starting treatment for primary nocturnal enuresis.
-Caution should be exercised in patients with other causes of urinary frequency (0.9. multiple sclerosis or urge incontinence), and in diabetes mellitus and renal impairment, since the use of desmopressin has not been well studied in these populations.
-Elderly patients and patients with low serum sodium levels may have an increased risk of hyponatraemia
-Precautions to avoid hyponatraemia must be taken in:
conditions characterised by fluid and/or electrolyte imbalances such as systemic infections, ever and syndrome of inappropriate ADH secretion (SIACH)
-conditions requiring concomitant treatment with diuretic agents
-concomitant treatment with drugs known to induce SIADH
-Treatment with desmopressin should be interrupted during acute intercurrent illness characterised by fluid and/or electrolyte imbalance (such as systemic infections, fever, gastroenteritis).
-Precautions must be taken in patients at risk of increased intracranial pressure.
-For each approved indication the lowest effective dose should be used.
Patient dosage should be reassessed periodically.
-Omegapress Tablets should be used with caution in patients with cardiovascular disease and the elderly.
-Omegapress Tablets should not be administered to dehydrated or overhydrated patients until water balance has been adequately restored.
-The risk of overhydration including cardiac failure should be borne in mind, especially in children or the elderly or in chronic use.
-Omegapress Tablets should be used with caution in patients with cystic fibrosis because of impaired water handling and increased risk of hyponatraemia.
Use in the elderly:
-The initiation of treatment in patients over 65 years of age is not recommended.
Pediatric use:
-Dose recommendations are the same as in adults. Children should be closely observed to avoid overingestion of fluid and to ensure that only the recommended dose of Omegapress Tablet is taken.
Pregnancy:
Caution should be exercised when prescribing to pregnant women.
Lactation:
desmopressin should not be used in breast feeding mothers.
Side Effects:
Treatment with and without concomitant reduction of fluid intake may lead to water retention/hyponatraemia with or without accompanying warning signs and symptoms (headache, nausea/vomiting, weight gain, and in severe cases, convulsions).
The risk appears to be dose-related and the elderly (>60 years) are at increased risk.
During clinical trials with desmopressin in diabetes insipidus the following adverse events have been reported more than once: headache, cold, weight gain, dizziness, sore throat, and depressed mood.
The most serious adverse reaction with desmopressin is hyponatraemia, which may cause headache, abdominal pain, nausea, vomiting, weight increase, dizziness, confusion, malaise, memory impairment, verigo, falls and in severe cases convulsions and coma. The cause of the potential hyponatraemia is the anticipated antidiuretic effect. The hyponatraemia is reversible and in children it is often seen
Cranial diabetes insipidus
During clinical trials with desmopressin in diabetes insipidus the following adverse events have been reported more than once: headache, cold, weight gain, dizziness, sore throat, and depressed mood.
Some possible reaction:
Headache, Affect, Lability, Aggression, Abdominal pain, Nausea, Vomiting, Diarrhoea, (HLT), Bladder and urethral symptoms, Oedema, Peripheral, (HLT) Anxiety, Symptoms Nightmare, Mood swings, Nervous system, Disorders, Somnolene, Vascular disorders, Hypertension, Irritability
Storage Method:
Store at temperature not exceeding 30 C.
Keep away from reach of children.
Package:
A carton box contains 3 blisters with 10 tablets in each and insert leaflet.
Tags, Keywords:
Active Ingredients:
No comments yet. Be the first to write one.
Your comment will be published after moderation.
You’ll receive a notification to your email when someone replies.
 Русский
Русский English
English عربي
عربي