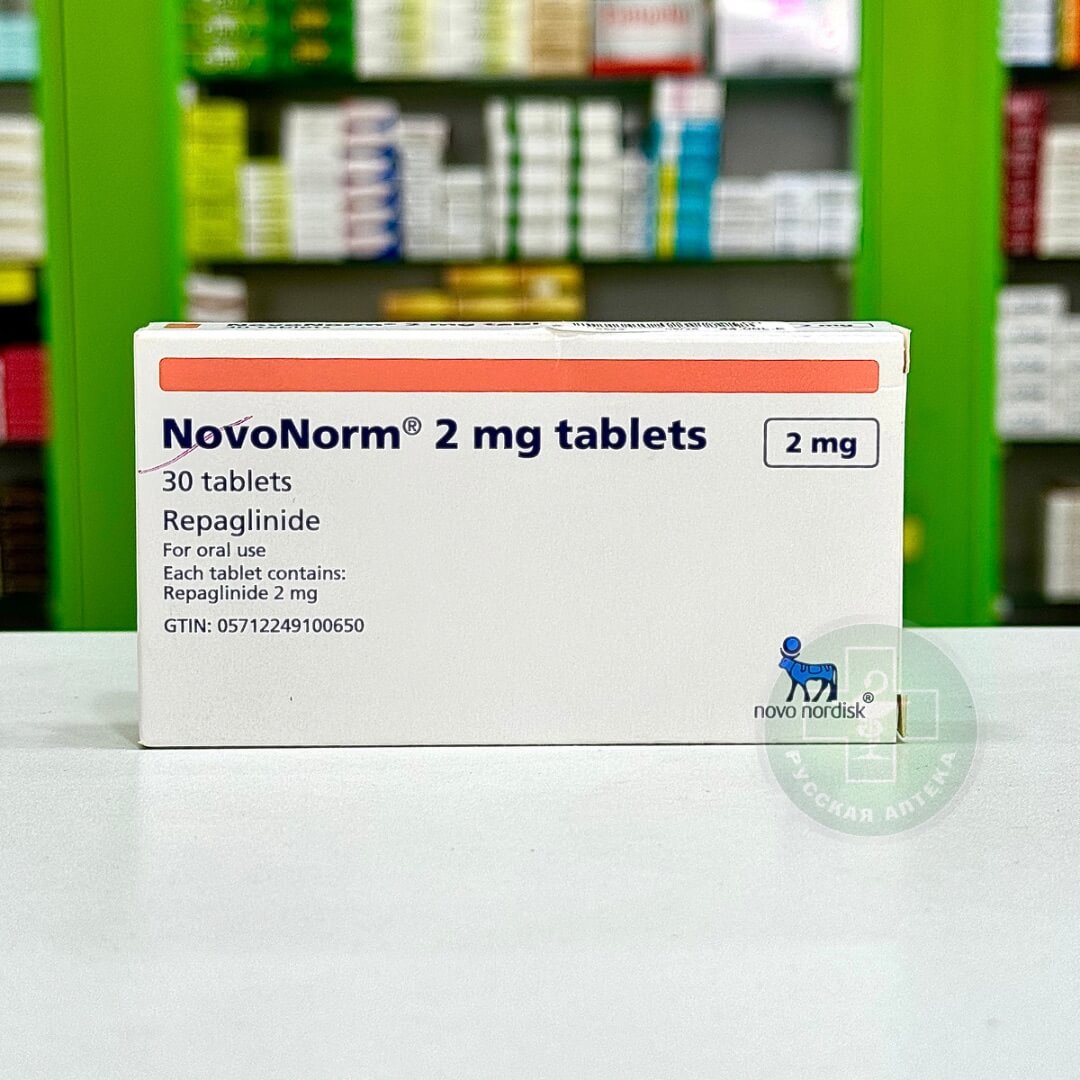
Empacoza Plus 25/5 mg 30 tablets
357£
View analogsAdults (≥18 years) with type 2 diabetes mellitus:
Inadequate glycemic control on metformin and/or sulphonylurea and one of the components of Empacoza Plus
Patients already treated with the free combination of empagliflozin and linagliptin
Buy
Product quantities
Form of Release: Tablets
Product Brand: Zeta Pharma
Product Categories: Diabetes
Empacoza Plus 5 mg/25 mg 30 Tablets
Composition
Each film-coated tablet contains:
Empacoza Plus 5/25 mg: Empagliflozin 25 mg and Linagliptin 5 mg
Description
Empacoza Plus is a fixed-dose combination of empagliflozin, a sodium-glucose co-transporter 2 (SGLT2) inhibitor, and linagliptin, a dipeptidyl peptidase-4 (DPP-4) inhibitor, indicated for adults with type 2 diabetes mellitus. It works by promoting urinary glucose excretion and enhancing incretin activity to improve blood glucose control. Empacoza Plus is intended for patients whose blood sugar levels are not adequately controlled with metformin and/or sulphonylurea and one of the individual components.
Indications for Use
Adults (≥18 years) with type 2 diabetes mellitus:
Inadequate glycemic control on metformin and/or sulphonylurea and one of the components of Empacoza Plus
Patients already treated with the free combination of empagliflozin and linagliptin
Dosage and Method of Administration
Starting dose: One tablet of 10 mg empagliflozin / 5 mg linagliptin once daily
If needed for better glycemic control: Increase to one tablet of 25 mg empagliflozin / 5 mg linagliptin once daily
Renal impairment: Do not initiate if eGFR or CrCl <60 mL/min; reduce dose or discontinue if eGFR or CrCl persistently <45 mL/min
Hepatic impairment: Not recommended in severe cases
Elderly: Use caution in patients ≥75 years old; not recommended for ≥85 years old
Contraindications
Hypersensitivity to empagliflozin, linagliptin, or any excipients
Type 1 diabetes mellitus
Diabetic ketoacidosis
End-stage renal disease or patients on dialysis
Precautions
Risk of diabetic ketoacidosis with atypical presentation
Monitor renal function regularly
Increased risk of hypoglycemia with insulin or sulphonylurea
Risk of acute pancreatitis, particularly in patients with history of pancreatitis
Monitor volume status in case of dehydration risk
Risk of urinary/genital infections
Possible hepatic injury or elevated hematocrit
Not recommended in patients aged ≥85 years
Pregnancy and Breastfeeding
Not recommended during pregnancy and breastfeeding due to lack of data
Side Effects
Very common: Urinary tract infections (7.5–8.5%)
Serious: Diabetic ketoacidosis (<0.1%), pancreatitis (0.2%), hypersensitivity (0.6%), hypoglycemia (2.4%)
Others: Genital infections, volume depletion, rash, hepatic enzyme elevations
Storage Conditions
Store below 30°C. Keep out of reach of children.
Packaging
Carton box contains 3 blisters of 10 film-coated tablets and a package insert.
Tags, Keywords:
Active Ingredients:
No comments yet. Be the first to write one.
Your comment will be published after moderation.
You’ll receive a notification to your email when someone replies.
 Русский
Русский English
English عربي
عربي