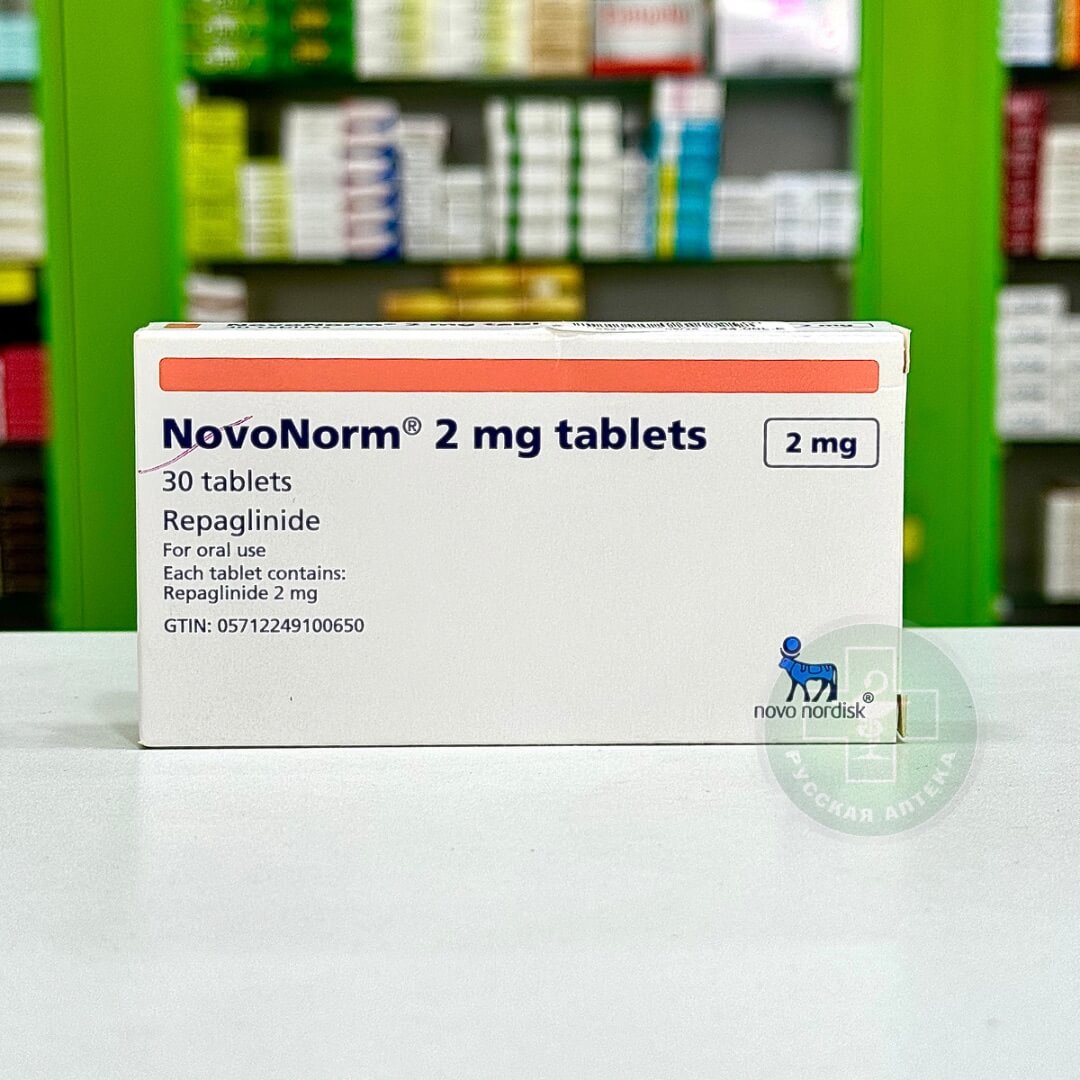
Icandra Plus 50/1000 mg
192£
View analogsType 2 diabetes mellitus (in combination with diet therapy and physical exercises).
Buy
Product quantities
Form of Release: Tablets
Product Brand: Mash Premiere for Pharmaceutical Industries
Product Categories: Diabetes
Trade Name:
Icandra Plus 50 mg/1000 mg
Composition:
Each tablet contains:
Vildagliptin 50 mg
Metformin hydrochloride 1000 mg
Inactive ingredients:
Microcrystalline cellulose, sodium croscarmellose, Povidone K30, Aerosil, magnesium stearate.
Properties:
Combined hypoglycemic agent for oral administration.
Vildagliptin, a representative of the class of stimulators of the pancreatic insular apparatus, selectively inhibits the enzyme DPP-4, improving glycemic control. Inhibition of DPP-4 activity causes an increase in both basal and postprandial endogenous levels of incretin hormones: glucagon-like peptide type 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP).
Metformin reduces glucose production by the liver, reduces glucose absorption in the intestine and reduces insulin resistance by increasing the uptake and utilization of glucose by peripheral tissues. Metformin induces intracellular glycogen synthesis by acting on glycogen synthetase, and enhances glucose transport by some glucose-carrying membrane proteins (GLUT-1 and GLUT-4). Improves glucose tolerance in patients with type 2 diabetes mellitus by reducing the concentration of glucose in blood plasma both before and after meals. Unlike sulfonylurea derivatives, metformin does not cause hypoglycemia either in patients with type 2 diabetes mellitus or in healthy individuals (except in special cases). Metformin does not lead to the development of hyperinsulinemia. When metformin is used, insulin secretion does not change, while the concentration of insulin in the blood plasma on an empty stomach and during the day may decrease.
The combination of these components makes it possible to more effectively control the concentration of blood glucose in patients with type 2 diabetes mellitus for 24 hours.
Indications:
Type 2 diabetes mellitus (in combination with diet therapy and physical exercises).
Method of administration and dosage:
The optimal dosage regimen is determined by the doctor. It is necessary to strictly observe the compliance of the used dosage form of a particular drug with the indications for use and the dosage regimen.
When using this combination, do not exceed the recommended maximum daily dose of vildagliptin 100 mg.
The maximum daily dose of metformin is from 500 mg to 2000 mg per day.
Contraindications:
Type 1 diabetes mellitus; severe renal insufficiency or impaired renal function with GFR <30 ml/min; acute conditions with a risk of developing renal dysfunction: dehydration (with diarrhea, vomiting), fever, severe infectious
diseases, hypoxia conditions (shock, sepsis, kidney infections, bronchopulmonary diseases); acute and chronic heart failure, acute myocardial infarction, acute cardiovascular insufficiency (shock), respiratory failure; liver dysfunction; acute or chronic metabolic acidosis (including diabetic ketoacidosis in combination with coma or without it); lactic acidosis (including in the anamnesis); should not be used 48 hours before surgical operations, radioisotope, X-ray examinations with the introduction of contrast agents and within 48 hours after their implementation; chronic alcoholism, acute alcohol poisoning; compliance with a low-calorie diet (less than 1000 kcal / day); pregnancy, lactation (breast feeding); hypersensitivity to vildagliptin or metformin or any other components of the drug.
Warnings and precautions:
Drugs containing metformin are recommended to be used with caution in patients over 60 years of age when performing heavy physical work, due to the increased risk of developing lactic acidosis in them.
In patients receiving insulin treatment, this combination cannot replace insulin therapy.
Regularly during treatment with the drug it is recommended to determine the biochemical parameters of liver function. If an increase in the activity of aminotransferases is detected, a repeat study should be conducted to confirm the result, and then regularly determine the biochemical parameters of liver function until they normalize. If the excess of AST or ALT activity is 3 or more times higher than the IOP is confirmed by repeated examination, treatment is recommended to be canceled.
Side effects:
From the nervous system: often – headache, dizziness, tremor. When using vildagliptin in combination with metformin in various doses, hypoglycemia was observed in 0.9% of cases (for comparison, in the placebo group in combination with metformin – in 0.4%).
From the digestive system: often – nausea, gastroesophageal reflux, dysgeusia; infrequently – diarrhea, flatulence; very rarely – hepatitis.
From the side of metabolism and nutrition: very often – decreased appetite; often – hypoglycemia; very rarely – lactic acidosis.
Infectious and parasitic diseases: very rarely – upper respiratory tract infections, nasopharyngitis.
General disorders and disorders at the injection site: often – chills.
From the skin and subcutaneous tissues: often – hyperhidrosis; very rarely – skin reactions (in particular, erythema, itching, urticaria).
From the musculoskeletal and connective tissue: often – arthralgia.
From the side of the vessels: infrequently – peripheral edema.
Storage:
In a cool, dry place at a temperature not exceeding 30 °C.
Package:
A carton box contains 1.2 or 3 blisters of 10 tablets, paper leaflet.
Tags, Keywords:
Active Ingredients:
No comments yet. Be the first to write one.
Your comment will be published after moderation.
You’ll receive a notification to your email when someone replies.
 Русский
Русский English
English عربي
عربي