Other brands
- All Brands
- 2M Whites Pharma
- Abbott Laboratories
- Acdima International Trading
- Acino Egypt
- Adare Pharmaceuticals
- Advocure
- ADWIA Pharmaceuticals
- Al Abdellatif Al Tarshouby Pharmacies
- Al Delta Pharmaceutical Trading Co.
- Al Esraa Pharmaceuticals
- Al Rowad For Pharmaceutical Industry
- Alba Pharma
- Alcon
- Alex Express Co.
- Alexandria Pharmaceutical Company
- AlfaCure Pharmaceuticals
- Alliance Pharmaceuticals
- Almirall Products-Spain
- Amoun Pharmaceutical Co.S.A.E
- Amriya for Pharmaceutical Industries S.A.E
- ِAlandalous pharmaceutical
- Apex Pharma
- Arab caps
- Arab Drug Company (ADCO)
- Arax Pharma
- Aspen Pharma Trading Limited
- Aspin Pharma
- Astellas Pharma Europe B.V
- AstraZeneca
- Atco pharma
- ATOS Pharma
- AUG Pharma
- Ausnutria Private Label B.V.
- Avano Pharma
- Averroes Pharma
- Avrelle Cosmetics
- B.P. Pharma
- Batterjee Factory Co. for Cosmetics Ltd
- Bausch + Lomb
- Bayer
- BBI Pharma
- Bellezza Pharma
- Bio Medical
- Bio Pharma Egypt
- Bio Soft
- BioMED
- Bionorica SE
- Laboratoires expanscience - France >Biotech Egypt
- Biotech Pharma
- Biotech Pharma LTD
- Bobana
- Boehringer Ingelheim
- Borg Pharmaceutical
- Brand International Pharma
- Brother Pharma
- Bubbles International
- Burda Bebek Ürünleri San. ve Tic. A.Ş
- CeraVe
- Chemipharm
- Chiesi Farmaceutici S.p.A
- CID Pharmaceuticals
- Cipla Ltd
- Cira pharma
- Cleopatra
- Colgate-Palmolive Company
- Copad Egypt
- CosmoTrade
- Dabur
- Danone
- Dawa Egypt
- DCS Group (UK) Ltd
- Debeiky pharma
- Delta Pharma
- Devart Lab Pharmaceutical Co.
- Diamond Pharma
- DKT
- Dr. Falk Pharma GmbH
- Dr. Rashel
- Dr. Wilmar Schwabe GmbH & Co.KG
- Drug Pharma Egypt
- Dulex Lab Pharmaceutical Company
- E.T. Browne & Co., Inc.
- ECCT - Egyptian Company for Cosmetics and toiletries
- Eckhart Corp.
- Edgewell Personal Care Company
- EGPI
- Egyphar
- Egypharma
- Egyptian Company for Cosmetics (E.C.C)
- Egyptian pharmaceutical trading company
- EIMC Pharma
- Eipico
- El Esraa Pharmaceutical Optima
- El Nasr
- El Nile
- El Sisi International co
- El-Obour
- Elerte-France
- Eli Lilly
- Elixir Pharma
- Elle Cosmetics
- Elretag Cosmetic
- Ema Pharma
- European Egyptian Pharmaceutical Industry
- Eva Cosmetics
- Eva Pharma
- Ever Neuro Pharm
- Evita For Cosmetics
- Excellence pharma group
- F. Hoffmann-La Roche
- Falcon Group
- Farouk Systems, Inc.
- Ferrer International S.A
- Fide
- Fine Hygienic Holding
- Fournier
- Fresenius Kabi
- Fuchs GmbH
- Future Pharma
- Genesis
- GeoPharma 2001
- Gerolymatos International S.A
- GlaxoSmithKline
- Glenmark Pharmaceuticals Egypt
- Global Advanced Pharma
- Global napi pharmaceuticals
- Global Pharmaceutical Industries (GPI)
- Globe International
- Glory Henna
- Golden Queen
- Good Care
- Gr. Sarantis S.A
- Grand Pharma
- Granty
- Guangzhou Livepro Beauty Cosmetics Co., Ltd.
- GYPTO Pharma
- Habib Scientific Office (HSO)
- Happy Sweat for Topix nutritients
- Hayat Egypt Hygienic Products
- Helian
- Henkel
- Hepta
- Hero Middle East & Africa
- Hexagon Pharmaceuticals
- Hi Pharm
- Hikma Pharmaceuticals
- Himalaya Wellness Company 1930
- Hochster Pharmaceutical industrie
- Hygint
- IBSA Switzerland
- Imtenan
- Incandesce pharma
- Infinity Clinic Pharma
- Ingram's
- Inspire Pharmaceutical Company
- International Drug Agency
- International Medical Guide (IMG)
- Interpharma
- ISC Pharmaceuticals
- Isis Egypt
- Isis Pharma
- J.Casanova
- Jamjoom Pharmaceuticals
- Janssen-Cilag
- Jedco International for Pharmaceuticals
- Jeil Pharmaceutical Co. Ltd
- Johnson & Johnson
- Johnson Wax Egypt
- Julphar
- Kahira Pharmaceuticals
- Karishma Cosmetics
- Kempetro for chemical industries
- Kimberly-Clark Corporation
- KMT
- Kyan Egyp
- L'Oréal
- La Roche-Posay
- Laboratoire Bioderma
- Laboratoire de la Mer>Star International company
- Laboratoire Dermatologique ACM
- Laboratoires Dermatologiques Ducray
- Laboratorios KIN
- Original Pharma
- Leap Cosmeceuticals
- LEO Pharma
- Lider Kozmetik
- Linco Care Ltd.
- Lion Wings
- Lipha Santhe
- Liptis Pharmaceuticals Factory
- Lotus
- Luna
- Lundbeck
- MACRO GROUP Pharmaceuticals
- Maddox Pharma
- Majestic BioPharma
- Mann & Schröder Cosmetics Group
- Mann & Schröder GmbH
- Marcyrl Pharmaceutical Industries
- Marnys
- Marvel Pharma
- Marvel Pharma Group
- Mash Premiere for Pharmaceutical Industries
- Master Business Group
- MDI Pharma
- Med Care
- Meda AB
- Medical Union Pharmaceuticals main planet MUP
- Medinza Pharm Cosmetics
- Medizen Pharmaceutical
- MEGA Disposables S.A.
- Melano pharma
- Memphis Pharma
- Menarini
- Mepaco
- Merck
- Merck Santé S.A.S
- Merz
- MG Pharma
- Minapharm Pharmaceuticals
- Mira International
- Misr Pharma
- Mobease Pharmaceuticals
- Mollinsy
- MOON Pharma
- Morfose Kişisel Bakım ve Kozmetik Ürünleri Sanayi Ticaret A.Ş.
- Multicare
- Multipharma
- Mundipharma
- Mylan SAS
- Nactalia International
- Namaste Laboratories
- Napco Pharma Industry
- Nascita
- Nerhadou International
- Nerhadou International co
- Nestle
- New Port-Ireland
- Next Pharma
- Nile for Cosmetics
- Nivea
- Nova Pharm
- Novartis
- Novell Pharma
- Novex
- Novo Nordisk
- NOW Foods
- NP Pharmaceutical
- Nutravia Pharma
- October Pharma
- Oli Paradise
- OLIS Trading
- Olivia
- OM Pharma
- Opal Egypt
- OPCI (Omega Pharmaceutical & Chemical Industries)
- Oralines
- Orchidia Pharmaceutical Industries
- Organix
- Organo Pharma
- Original Pharma
- Original Pharma Group
- Oson Cosmetics
- Otsuka Pharmaceutical
- Panax Pharma
- Par Par
- Parkville Pharmaceuticals
- Paxal Pharmaceuticals
- PDC Brands
- Penta Pharma
- Performance Health
- Pfizer
- Pharco
- Pharco B
- Pharco Pharmaceuticals Inc.
- Pharma - Credo
- Pharma Cure Pharmaceuticals
- Pharma Life
- Pharma zad
- Pharmacare
- Pharmachem
- Pharmacon
- Pharmantura S.p.A.
- Pharmaplast
- Pharo Pharma
- Phoenix Group
- Pierre Fabre
- Pixel Pharmaceutical Pvt Ltd
- Planet Cure Pharmaceutical Company
- Pontagar
- Positive Cosmetics LTD
- Premier Pharmaco
- PRIMA
- Procter & Gamble
- Purex Health Care
- Pyramids Cosmetics
- Queen Pharm International
- Ramco
- Rameda Pharmaceutical Company
- Ranbaxy
- Reckitt Benckiser
- Riker england
- Roche
- Roofa
- Roventis Pharma
- Royal Regime
- Sabaa Pharmaceuticals
- Safe Pharma
- Safу life pharma
- SAJA Pharmaceuticals
- Sandoz
- Sanita Consumer Products
- Sanofi Egypt
- Sanosan GmbH
- Santom
- SC Johnson
- Schering-Plough
- Sedico Pharma
- Seif Pharmacies
- Sekem
- Semak
- Serolam France
- Servier
- Servilife Pharma
- Seutic Pharma
- SFN Pharma
- Sigma
- Sigmatec
- Smithkline Beecham
- Soficopharm
- Soficopharm
- Solovit
- Sovalue Pharma
- Sphinx Pharmaceuticals LTD
- Spimaco Misr
- Splendid pharma
- Stada
- Stafford-Miller (Ireland) Limited
- Star International
- Starville
- Steady Pharma
- Sun Bright
- Sun Pharmaceutical Industries Ltd.
- Synthon
- Tabuk Pharmaceuticals
- Tadawena
- Tag Pharma
- Talent Pharma
- Technopharma
- Teijin Limited Japan
- Tempo free extra
- Tetrapharm
- The Egyptian Saudi Co. For Medical Manufacturing (Masco-Mid) S.A.E,
- Thornton & Ross Limited
- TNG Cosmetic Industries
- Trend Pharm
- Triscom B.V.
- U.P. PHARMA
- UGC Pharma
- UK Medical Limited
- Unicharm Corporation
- Unilever
- UNIPHARMA CO.
- Utopia Pharmaceutical Company
- Vasu Healthcare Pvt Ltd
- Viatris
- VICHY
- Vitabiotics
- Vortex pharma
- Walfarm
- Warnex Pharma
- Wassen International Ltd
- Western Pharmaceutical Industries
- Western Pharmaceutical Industries
- Wockhardt
- Wyeth Ayerst Lederle-Canada-Viatris Egypt
- X Factor medical
- Xeedia Pharma
- ZAD Industrial Pharma
- Zambon
- Zeta Pharma
- Zoser Pharma
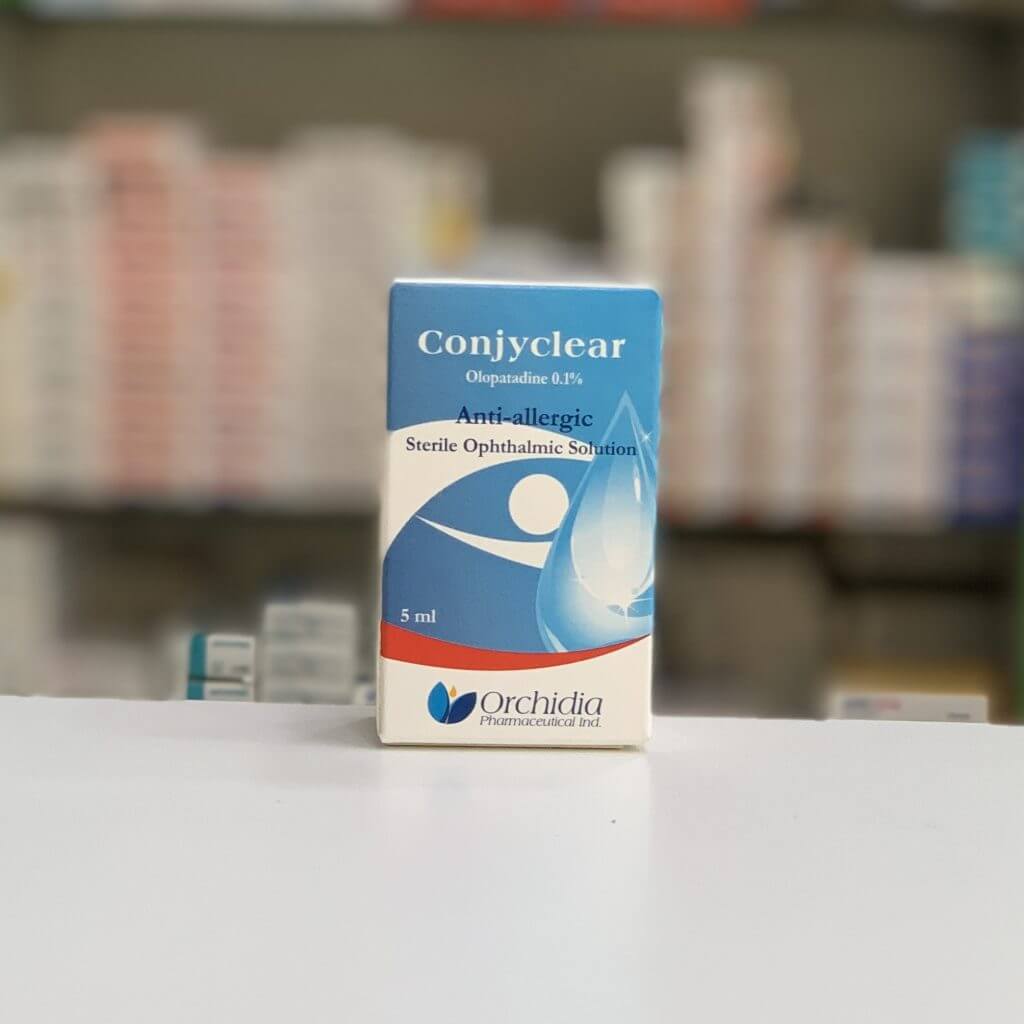
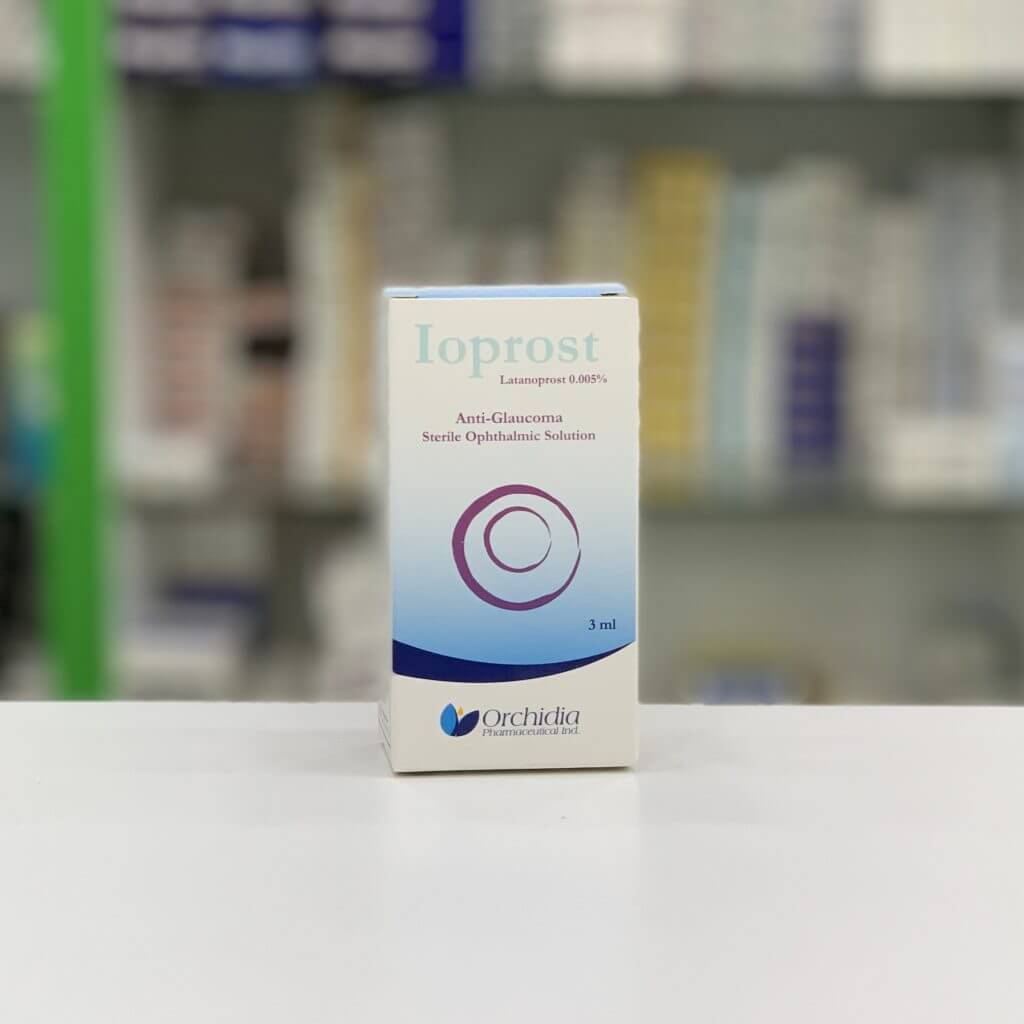
Orchidia Pharmaceutical Industries

By 2020, Orchidia expanded its production of cosmeceuticals and nutraceuticals through the introduction of premium products and advanced technologies.
Orchidia is certified for WHO-GMP implementation by the Egyptian Drug Authority (EDA) and other regulatory bodies in the Middle East and Africa. It also holds ISO 9001:2015, ISO 14001:2015, ISO 45001:2018, ISO 17025:2017, ISO 13485:2016 certifications, as well as the European CE marking. The company continuously maintains and improves its Quality Management Systems (QMS) and Environmental, Health, and Safety (EHS) practices to achieve the required quality standards and exceed stakeholder expectations.
Its current portfolio includes 60 ophthalmic products and 11 medical devices, 8 of which are CE certified. In addition to a wide range of various ophthalmic products in different pharmaceutical forms, Orchidia’s CE-marked products are registered with the Belgian Ministry of Health by SGS Belgium 1639 and represented in Germany by MDSS (Medical Device Safety Services).
Orchidia’s products are available beyond national borders in 40 countries, primarily in the Middle East and Africa regions. The company is also in the process of registering in several additional countries.
 Русский
Русский English
English عربي
عربي