Tradename:
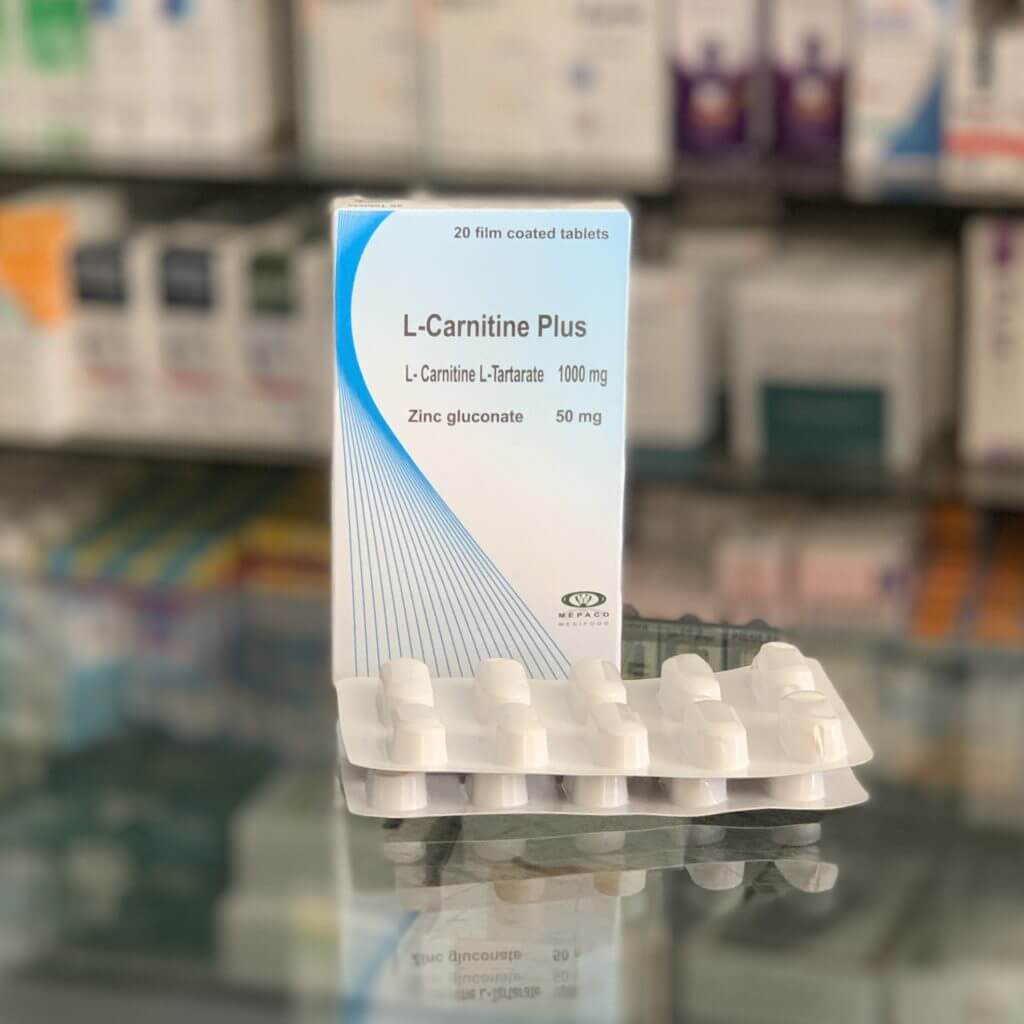
Carnivita forte
Compound:
Each p/o tablet contains:
L-carnitine tartrate – 1000 mg.
Zinc gluconate – 50 mg.
Auxiliary components: corn starch, polyvinylpyrrolidone, microcrystalline cellulose, sodium stearyl fumrate, croscarmellose sodium, anhydrous colloidal silicon, white opadrium.
Properties:
L-carnitine is an important cofactor that has a beneficial effect in various pathologies affecting both vascular and muscle tissues. It is also an amino acid derivative of lysine and methionine; and an important factor in fatty acid metabolism in skeletal muscle, heart, and liver. L-carnitine is concentrated in tissues, about 97% in the skeleton. In the muscle cell, L-carnitine is the molecule that transports long-chain fatty acids across the mitochondrial membrane that stimulate energy production, so L-carnitine is considered a natural metabolic modulator as it is an important factor for fatty acid oxidation in cellular mitochondria.
Zinc is a mineral that is used in minute amounts in almost every cell in the human body. It is necessary for the proper functioning of the nervous and optimal response of the immune systems. Zinc also plays a special role in the reproductive process, its crucial role being played in the development of sperm in men and eggs in women.
Indications:
primary carnitine deficiency and secondary carnitine deficiency in adults and children over 12 years of age.
Mode of application:
Adults and children over 12 years of age: Tablets should be given in divided doses. In congenital errors of metabolism: the required dose depends on the specific inborn error of metabolism and the severity of the manifestation during treatment. Generally recommended dosage: Up to 200 mg/kg/day in divided doses (2 to 4) is recommended for chronic use in some disorders, with lower doses sufficient for other conditions. If symptoms do not improve, the dose may be increased in the short term. Higher doses up to 400 mg/kg/day may be necessary in acute metabolic decompensation. Hemodialysis – maintenance therapy: if the first course of intravenous carnitine has produced significant clinical benefit, maintenance therapy using 1000 mg oral Carnivit per day may be considered. It should be administered on the day of dialysis at the end of the session.
Contraindications:
hypersensitivity to any component in the composition.
Side effects:
with long-term use of oral levocarnitine, various mild gastrointestinal complaints have been reported: nausea and vomiting, abdominal cramps and diarrhea. Reducing the dose often reduces or eliminates the patient’s body odor or gastrointestinal symptoms. The condition should be monitored very carefully during the first week of use and after any increase in dosage.
Pregnancy and lactation: no experience in pregnant women with primary systemic carnitine deficiency. Levocarnitine is a normal component of breast milk. The use of levocarnitine supplements in breastfeeding mothers has not been studied.
Precautionary measures:
administration of L-carnitine to diabetic patients may lead to hypoglycemia. The safety and efficacy of oral L-carnitine have not been evaluated in patients with renal insufficiency. Chronic administration of high doses of oral L-carnitine to patients with severely impaired renal function or patients with end-stage renal disease on dialysis may lead to the accumulation of potentially toxic metabolites, as they are usually excreted in the urine. This situation was not observed after intravenous administration of L-carnitine. Consult your physician before use if you are taking minocycline, doxycycline, tetracycline, or are on coumadin therapy, zinc treatment may interfere with the absorption of these medications.
Storage method:
Store at a temperature not exceeding 30C in a dry place out of the reach of children.
Package:
a cardboard box contains 1,2,3 blisters of 10 p / o tablets each, paper instructions.
 Русский
Русский English
English عربي
عربي