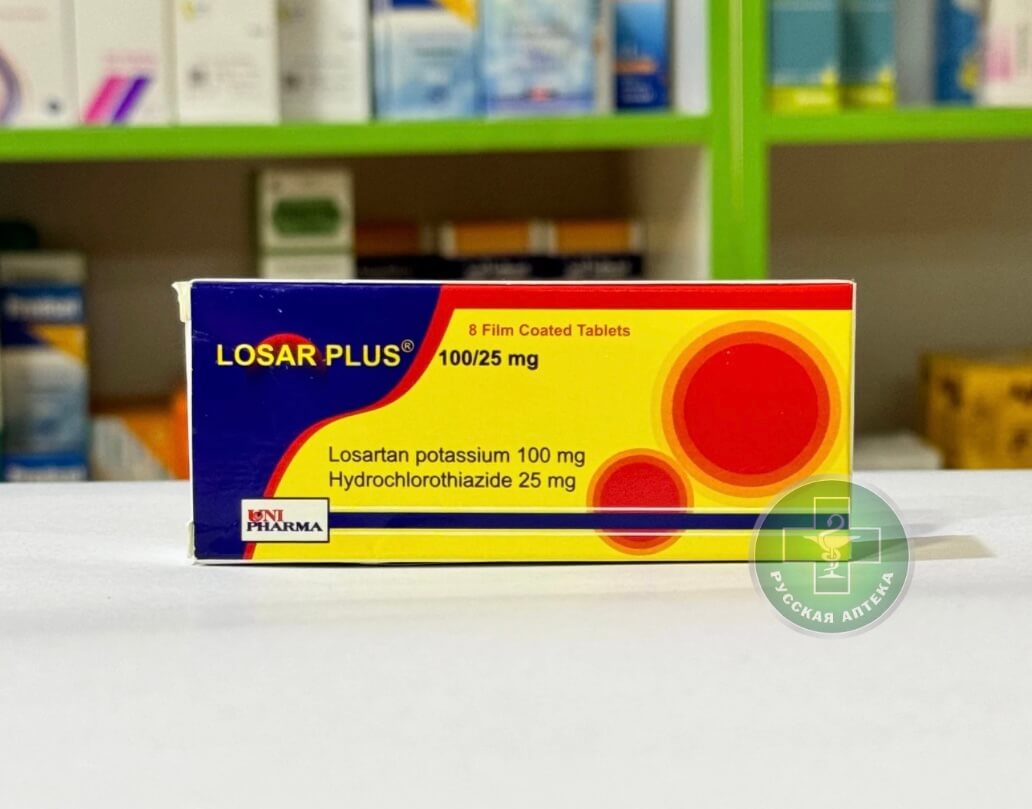
Losar Plus 100/25 mg 8 Film-Coated Tablets
Composition
Each tablet contains:
Losartan potassium — 100 mg
Hydrochlorothiazide — 25 mg
Description
A fixed-dose combination of an angiotensin II receptor blocker (losartan) and a thiazide diuretic (hydrochlorothiazide), designed for patients with essential hypertension not adequately controlled by either component alone.
Indicated for the treatment of essential hypertension in patients not adequately controlled with losartan or hydrochlorothiazide alone.
Indications for Use
Treatment of essential hypertension in patients whose blood pressure is not adequately controlled on monotherapy with losartan or hydrochlorothiazide.
Dosage and Method of Administration
Tablets should be swallowed with water, with or without food.
Not recommended as initial therapy.
Dose titration with individual components is advised.
Usual maintenance dose: 1 tablet of Losartan 50 mg / HCTZ 12.5 mg once daily.
If needed, dose can be increased to 1 tablet of Losar Plus 100/25 mg once daily (maximum dose).
Full antihypertensive effect usually achieved in 3–4 weeks.
Special Populations:
Renal impairment: Contraindicated if creatinine clearance <30 ml/min.
Hemodialysis patients: Not recommended.
Hepatic impairment: Contraindicated in severe hepatic dysfunction.
Children and adolescents (<18 years): Not recommended.
Contraindications
Hypersensitivity to losartan, hydrochlorothiazide, or any excipients
Severe renal impairment, anuria
Severe hepatic impairment
Refractory electrolyte disturbances (hypokalemia, hypercalcemia, hyponatremia)
Symptomatic hyperuricemia/gout
Pregnancy and breastfeeding
Concomitant use with aliskiren in patients with diabetes or impaired renal function (GFR <60 mL/min/1.73 m²)
Precautions
Do not initiate during pregnancy. Discontinue immediately if pregnancy occurs.
Risk of symptomatic hypotension in volume- or sodium-depleted patients (e.g., diarrhea, vomiting, salt restriction).
Monitor serum electrolytes (especially potassium) and renal function regularly.
Increased risk of hyperkalemia when used with potassium-sparing diuretics, supplements, or salt substitutes.
Risk of photosensitivity and non-melanoma skin cancer — advise sun protection.
Possible psychiatric effects (insomnia, anxiety, depression), exacerbation of systemic lupus erythematosus.
Contains lactose — contraindicated in patients with lactose intolerance, galactosemia, or glucose-galactose malabsorption.
Side Effects
Common: Headache, dizziness, cough, fatigue, muscle cramps, dyspepsia, hyperkalemia
Rare: Angioedema, hyponatremia, elevated ALT, hypotension
Very rare: Hepatitis, rhabdomyolysis, photosensitivity, skin reactions
Hydrochlorothiazide: Nausea, vomiting, photosensitivity, electrolyte imbalance, hyperlipidemia, rash, decreased libido, elevated uric acid
Pregnancy and Lactation
Pregnancy: Contraindicated. May cause fetal injury or death.
Breastfeeding: Not recommended. May reduce milk production. Use alternative treatments.
Storage Conditions
Store below 30°C in a dry, dark place. Keep out of reach of children.
Packaging
Carton box containing 1 blister strip of 8 tablets and a patient information leaflet.
 Русский
Русский English
English عربي
عربي